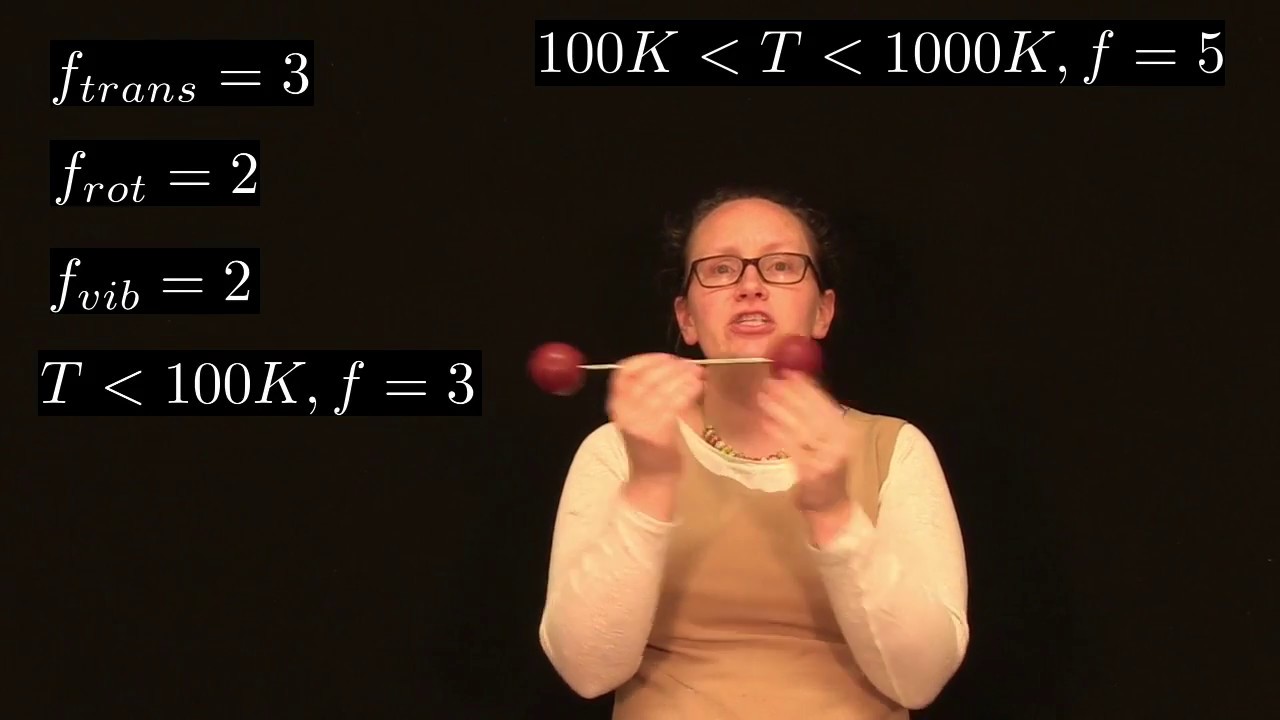Sounds good. I never really have mastered the fundamentals.
Let me start with the molecular level based on something I read in the first chapter of A Brief History of the Earth’s Climate by Steven Earle.
What he says, something I hadn’t previously seen mentioned, is that greenhouse gases (the major ones being water vapour, carbon dioxide, methane, nitrous oxide, and ozone: H2O, CO2, CH4, N2O, and O3) differ from the main gases of the atmosphere, nitrogen and oxygen (N2 and O2), in their molecular symmetry. There is energy in molecules in the way that the constituent atoms move with respect to each other (vibrational kinetic energy). The assymetry of the greenhouse gases give them two modes of vibration: 1. stretching back and forth along the axis of each bond and 2. bending or wobbling the atoms back and forth while holding the distance to the large atom fixed. The diatomic non-greenhouse gases only have the first mode of vibration. I’ll share a couple youtube videos to visualize this, but first an analogy…
Think of lifting two dumbbells. Your body is the carbon atom, the dumbbells are the oxygen atoms, and your arms are the bonds between the atoms. For H2O or N2O substitute different letters. For methane (NH4) I guess we need to also use our legs. A stretching vibration is like a shoulder press. The weights (oxygen atoms) change their distance from your body over each rep. The bending vibration is like a fly. You start with arms out to the side and bring the weights close together without changing the distance of them from your body.
For diatomic molecules (N2, O2) picture the dumbbells (atoms) as tied together by a rope (chemical bond) flying through space. The weights could move towards and away from each other along the axis of the rope, but there’s no equivalent to the fly exercise here. You could spin them along various axes of rotation, or you could move them through space together in each of the three spacial directions, but these are other kinds of kinetic energy (rotational and translational) not the vibrational kind.
Steven Earle says that the stretching vibration is higher frequency than the frequency of either visible light or infrared radiation. So visible light (plus some ultraviolent – there’s another case of a certain frequency of radiation being absorbed by a certain kind of molecule but on the way in instead of when heading back out) passes through the atmosphere unimpeded and is absorbed by the ground (some of it is reflected back, depending on the material – this (albedo) will be important to later discussions). The ground warms up as a result. Warmer things emit more infrared radiation. Some of that infrared radiation is directed upwards towards space.
Now we get to the other vibration mode: bending. Earle says this is lower frequency and happens to match that of infrared radiation. Because the frequencies match, the infrared energy gets used up increasing the bending vibrations in the various greenhouse gas molecules (that’s a bit hand wavy – would be interesting to learn the physics there) instead of escaping into space. That extra energy instead is retained in the atmosphere or transmitted from there by some means into the ocean, increasing the total energy available in earth systems ready to be deployed as different climate effects than what we had before the increase. Later he mentions that there is satellite evidence of an infrared gap in the spectrum of what escapes to space.
Btw. it seems to me I’ve read that the ocean holds a lot more heat than the air and has increased in temperature in a way that’s more directly correlated with CO2 increases. I mean, CO2 increases are very regular, but the temperature record is a little weird. From about 1980 on there’s quite a clear increasing trend. But mid-20th century not so much.
Links I promised:
Visualization of the vibrational kinetic energy in molecules:
A bit past four minutes in here she shows a play diatomic molecule and shows vibration along the axis of its bond a little later. Doesn’t mention the bending vibrational mode.
The following lecture describes three vibrational modes for CO2
- symmetry stretching mode
- asymmetry stretching mode
- bending mode
Asymmetry (asymmetrical?) mode just means that the bouncing back and forth is out of sync between the two molecules, i.e. one is closer to the big atom when the other is farther away.
Carbon dioxide vibration modes (CO2 molecule) - YouTube
Animations of above: https://www.youtube.com/watch?v=FC_NX2xPfvg